One of the most
important scientific arguments in favor of totally raw diets, and uncooked
foods in general, is that the proteins in foods are denatured by the high
temperatures of cooking.
Unfortunately, this powerful concept is
obscured and confused by an ancient book by Edward Howell, titled Enzyme
Nutrition, wherein he speculates that: "I adhere to the philosophy
[Note: philosophy, not science - ljf] that both the living organism
and its enzymes are inhabited by a vital principle or life energy which
is separate and distinct from caloric energy". "The enzyme
complex harbors a protein carrier inhabited by a vital energy factor.",
yet he can not demonstrate any existence of this mysterious "life
energy", nor describe how to detect and measure it. This unsupportable
metaphysical concept was actively propagated by the old Hippocrates Health
Institute in Boston in the 1970's and it still plagues and embarrasses
the raw food community to this very day.
How do we know if something is alive? The
best test I have come up with is that it must eat, excrete, and reproduce.
Another quality might be the ability to self-repair. Clearly,
enzymes, which are merely proteins, do not manifest any of these properties
unique to living beings.
Enzyme Nutrition still sells, and
holds the Amazon.com Sales Rank: 29,980 as of June, 2003.
The back cover of the book states: "In
1930, Dr. Howell established his own facility for the treatment of chronic
ailments, ..." and he retired in 1970, so, clearly, his concepts
are over 75 years out of date! If information doubles every ten
years, there is now available ~ 180 times as much information today as
when Howell made these speculations. Certainly, IF enzymes were
really "alive", it would be well known today.
He further claims: "... the capacity
of living organisms to make enzymes ... is limited and exhaustible"
quite to the contrary of modern biochemistry.
The claim that the enzymes in "foods"
help in the foods' own digestion is nonsensical, as plant enzymes are
quite specific to individual chemical reactions supporting the plant's
biochemistry, and they certainly do not "know" anything about
human digestive biochemistry and they have not changed their biochemistry
for our digestive convenience. Since plant species tend to be enormously
older than our little psychotic ape species, just how did the plants anticipate
human evolution and graciously alter their own biochemistry for our convenience?
Further, enzymes generally operate within
very limited ranges of temperatures and pH (acidity) and since the human
stomach tends to be very acidic, whereas plant sap is not, plant enzymes
would not be active in the human stomach. Finally, enzymes are proteins,
so wouldn't they be digested the same as other food proteins, thus destroying
their enzymatic action?
So, what does
the heating and consequent denaturing of proteins really do?
Proteins
are composed of strings of amino acids arranged in quite specific orders,
like beads on a string . This linear structure is the primary
structure. However, these strings of amino acids are then folded
upon themselves by relatively weak hydrogen bonds (proton bonds) into
complex three-dimensional structures
having secondary,
tertiary,
and quaternary
"higher order" structures. The biological activity
of molecules is determined by these higher three-dimensional structures
and how they physically fit into the three-dimensional structures of other
biomolecules. Since strict physical conformance of the enzyme
to the food protein it acts upon (say, in digesting it) must be satisfied
for the bioactivity, this tight complementary relationship is frequently
referred to as a "lock and key" model. Destroy
these higher structures and the chemical most probably becomes biologically
inactive: bend the key and it will not open the lock. Denaturation
is a process that alters a proteinís native conformation and biological
activity. If the tertiary or quaternary structure of a protein is
altered,
e.g., by such physical factors as extremes of temperature, changes in
pH, or variations in salt concentration, the protein is said to be denatured;
it usually exhibits reduction or loss of biological activity.
[A good primer
on protein folding, and an opportunity
to donate some of your unused computer cycles to a research project.]
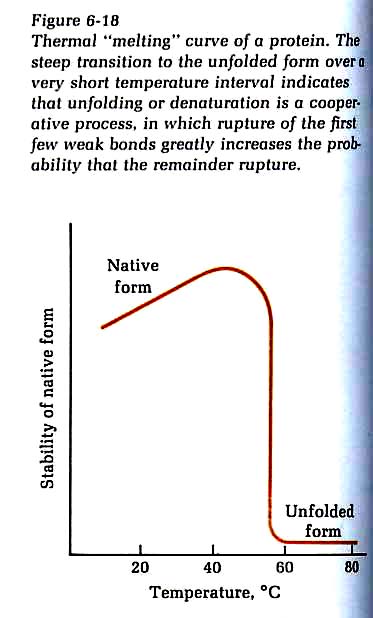
Lehninger, Biochemistry, 2ed., 6th printing, 1981, p. 144
Here,
we see that the higher structures of the protein in question collapse
at ~57°C (135°F: about 15F° higher than
the hottest water my finger can tolerate). It seems we have a built
in safety function mediated by pain sensors that forces us to remove body
parts from high temperatures before our structural proteins can be denatured
and rendered biologically-inactive: i.e. dead. The denaturing of
brain proteins and consequent loss of their functionality is the reason
medical personnel are very concerned about high fevers.
Pure water boils at sea level at 212°F,
deep frying oil is about 350-375°F, home stoves can broil at
air temperatures up to 500°F, with the surface temperatures
of the food even higher as a result of direct radiation from the heating
elements, and charcoal broiling can produce temperatures up to 700°F.
The USDA
recommends to cook hamburgers to 160°F internal temperature
as the "safest cooking method", the surface temperatures would
obviously be much higher. So, normal cooking practices do indeed
heat proteins well above the temperatures at which they become denatured.
Since both cooking and stomach acid denatures
proteins, it would be quite informative and necessary to determine if
both processes result in exactly the same unfolding; however, this
is rather unlikely as different mechanisms are operating. Since
the human digestive system uses several different proteolytic enzymes,
and protein digestion occurs in several quite separate and distinct steps,
the correct three-dimensional structures must be presented to each
enzyme for each step to be successful, in the proper order. If
cooking does not produce exactly the same physical structures as
acid denaturing, the first digestive step will be incorrect. Thus,
it will not present the proper structures for the second step and the
entire sequential digestive process is disrupted and distorted.
The fact that meat-eaters' feces have offensive,
and highly-characteristic, odors in the form of toxic amines, such as
indole, skatol, indican, putrescine [NH2(CH2)4NH2],
and cadaverine [NH2(CH2)5NH2]
(all from tryptophan),
neurine, and ptomatropine, is
clear evidence that their proteins were not digested properly, for if
they had been, there would be no amines, or residual nitrogen, for the
putrefactive bacteria in the colon to metabolize to produce these odors.
Properly digested proteins would produce no offensive amine compounds,
since all the protein would have been digested to amino acids which are
absorbed into the body, leaving none in the colon to support the putrefactive
bacteria.
"As the diet changes, the intestinal
flora also changes.
Gram negative anaerobes are observed when meat is introduced into
the diet. Persons who consume meat exhibit proportionately higher
numbers of Bacteroides and other gram negative anaerobes in comparison
with those on a vegetarian diet." Thus, as in all ecosystems,
the species that predominate are a direct function of the foods available.
In addition to denaturing, the high temperatures
of cooking causes cross-linking of some proteins, which makes them hard,
such as eggs or breads
getting harder on cooking, and that also profoundly reduces or eliminates
the ability of these proteins to be properly digested.